The North America aortic stent graft market is on a growth trajectory, valued at USD 3.1 billion in 2023 and expected to reach USD 5.2 billion by 2032. This growth is driven by factors such as the aging population, advancements in medical technology, and an increasing focus on minimally invasive procedures. In this blog, we delve deep into the market dynamics, segmentation, competition, and future prospects.
1. Understanding the Market Landscape
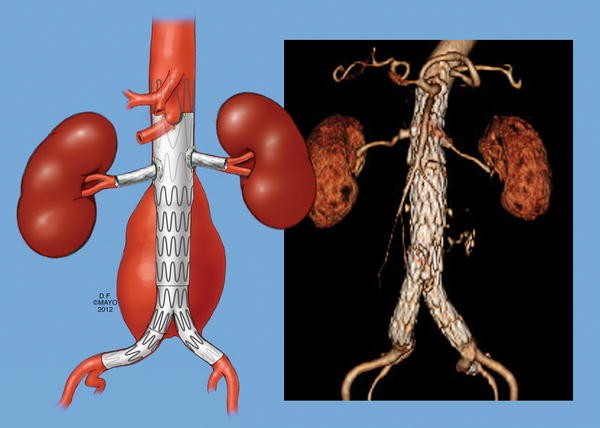
Aortic stent grafts are vital in treating aortic aneurysms, a potentially life-threatening condition. These devices are used in endovascular aneurysm repair (EVAR) procedures to reinforce the weakened section of the aorta and prevent rupture.
In North America, the market has witnessed significant growth due to advancements in materials and designs that enhance device flexibility, durability, and patient outcomes. By 2032, the market is projected to grow at a compound annual growth rate (CAGR) of 5.8%, indicating robust demand and innovation in this space.
2. Key Market Drivers
Several factors are propelling the market forward:
Aging Population
As the North American population ages, the prevalence of aortic aneurysms is increasing. Older adults are more susceptible to vascular conditions, making the adoption of stent graft solutions critical in improving survival rates and quality of life.
Technological Innovations
Advances in stent graft design and materials have revolutionized treatment. Features like improved flexibility, biocompatibility, and integration with imaging technologies allow for precise placement and better outcomes.
Shift Toward Minimally Invasive Techniques
Endovascular aneurysm repair (EVAR) is gaining traction over traditional open surgeries due to its minimally invasive nature, reduced recovery times, and lower risks. This trend is driving the demand for cutting-edge aortic stent grafts.
3. Market Challenges
Despite its promising outlook, the market faces challenges:
High Costs
Advanced stent graft devices come with significant costs, which may limit accessibility for some patients and healthcare systems.
Regulatory Barriers
Stringent approval processes by regulatory bodies like the FDA and Health Canada can slow down the introduction of new technologies. Manufacturers must navigate these hurdles while ensuring compliance with quality standards.
4. Market Segmentation
By Type
- Thoracic Aortic Stent Grafts: Designed for aneurysms in the thoracic region.
- Abdominal Aortic Stent Grafts: Used for abdominal aortic aneurysms, representing a significant market share.
By End-User
- Hospitals and Clinics: The primary settings for EVAR procedures.
- Ambulatory Surgical Centers: Emerging as a cost-effective alternative for outpatient procedures.
By Country
- United States: The largest contributor to the market, driven by advanced healthcare infrastructure and a high prevalence of vascular diseases.
- Canada: Demonstrating steady growth due to increasing healthcare investments and awareness.
5. Competitive Landscape
Leading Companies
The market features a mix of established players and innovators, including:
- Cook Medical, Inc.
- W.L. Gore & Associates.
- Medtronic Plc.
- MicroPort Scientific Corporation Inc., among others.
Strategic Initiatives
- Patent Analysis: Companies are investing heavily in research and development, securing patents for novel technologies.
- Partnerships and Collaborations: Collaborations between device manufacturers and healthcare providers are driving product adoption.
- Mergers and Acquisitions: Consolidation within the industry is enabling companies to expand their market presence.
6. Trends in Technology and Investment
Innovative Materials
Developments in biocompatible and durable materials are enhancing the performance of stent grafts.
Imaging Integration
New technologies that combine stent grafts with imaging systems allow for precise placement, reducing risks during procedures.
Funding and Investments
Venture capital is increasingly flowing into the medical device sector, with significant funding directed toward aortic stent graft innovations.
7. Regulatory Environment
The regulatory landscape in North America is stringent, ensuring patient safety but also posing challenges for manufacturers. FDA approvals and compliance with ISO standards are critical for market entry. Companies are investing in regulatory expertise to streamline product launches.
8. Future Outlook
The North America aortic stent graft market is poised for sustained growth, reaching a valuation of USD 5.2 billion by 2032. Key growth factors include:
- Continuous advancements in device technology.
- Increased adoption of minimally invasive treatments.
- Expansion of healthcare services across underserved regions.
As the market evolves, stakeholders must focus on innovation, affordability, and regulatory compliance to maintain a competitive edge.